Bond Angle of Cyclohexane
The Baeyer Theory and the Experimental Evidence of Ring Strain. A planar structure for cyclohexane is clearly improbable.

Convert Newman Projection Of Cyclohexane To Bond Line Chemistry Lessons Chemistry Textbook Study Chemistry
The boat conformation comes from partial C-C bond rotations only flipping one carbon up to convert the chair to a boat of the chair.
. What is the bond angle of Cycloheptane. The molecular formula of cyclohexane is C6H12. This bond angle keeps the carbon atoms as.
A planar structure for cyclohexane is clearly improbable. In cyclohexane all the carbon atoms are in a tetrahedral geometry with sp3 hybridization. So the C-C-H angles will be almost exactly 1095 degrees.
Cyclohexane Conformation Carbon atoms like to form bond angles of 1095 degrees. Answer 1 of 2. If the carbons of a cyclohexane.
Also every carbon-hydrogen bond in such a. In a typical planar hexagon the bond angles around any given carbon would be 120. Figure 43a Boat conformation of cyclohexane.
Bond angles are formed between two bonds with an atom in. Bond Angle of Cyclohexane. What are the bond angles of cyclohexane.
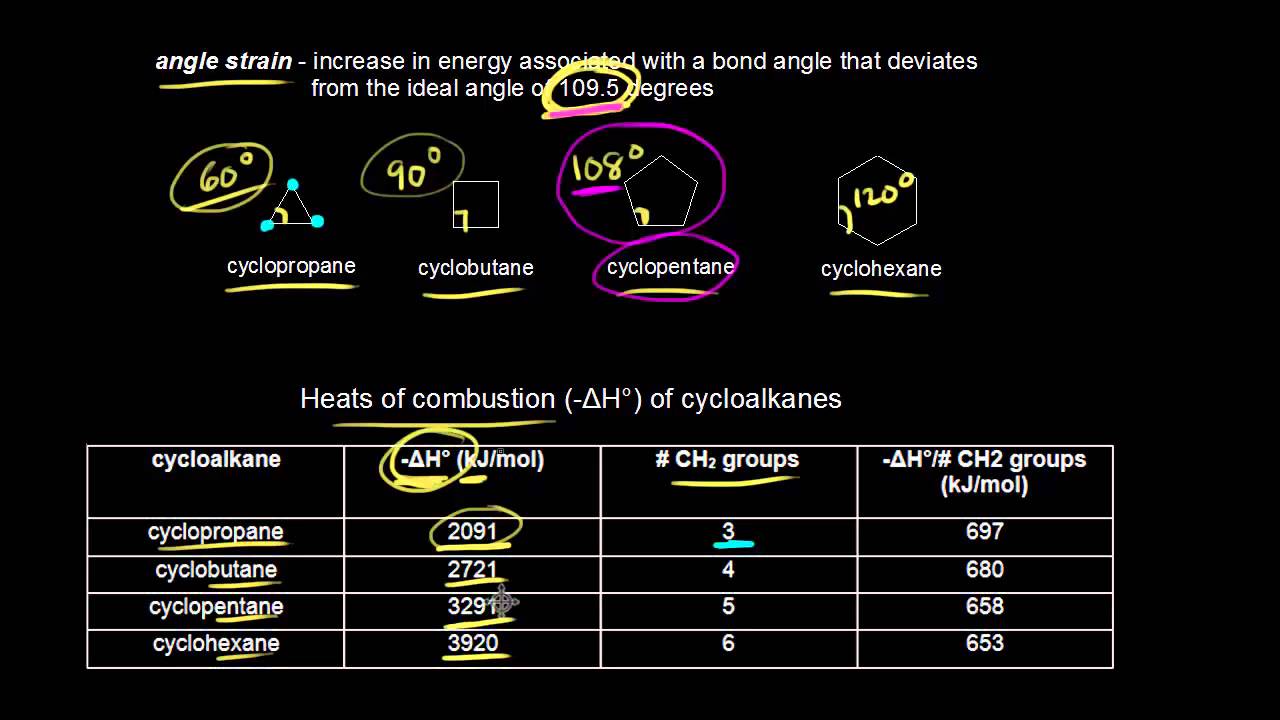
The bond angles would necessarily be 120º 105º larger than the ideal tetrahedral. Its stability results from the elimination of angle strain all bond angles are 1095 and torsional strain all groups on. So the angle between the C-H bond and the C-C bond is 1095 degrees in both case and I can derive the other sidesangles with sinecosine rules.
The bond angles would necessarily be 120º 105º larger than the ideal tetrahedral angle. 8-3-9 1069 In 1894 Baeyer. In the chair form of cyclohexane the carbon atoms and the bonds around them are almost perfectly tetrahedral.
See below for all values and xyz coordinates to read in with a molecular viewer. Compound n Angle Strain at each CH2. Answer 1 of 2.
Conformations of Cyclohexane. What is the bond angle of cyclohexane. A stable conformation adopted by cyclohexane.
If cyclohexane has a planar structure then the bond angles would be 120. Cyclohexane is one of the most important cycloalkanes and so is the focus discussion of the conformations of cycloalkanes. By forming a warped hexagon the bond angles become the ideal 1095 degrees.
But if cyclohexane were in a flat hexagon the bond angle would be 120 degrees. This deviation in bond angle from the ideal bond angle 1095. Values have quite a wide range angleceCCHapprox 100-120circ.
If cyclohexane has a planar structure then the bond angles would be 120. A regular hexagon shape. The molecular formula of cyclohexane is C6H12.
Cyclopentane has bond angles very close to 1095 but because 4 of its carbons are planar there it has torsional strain that makes it less stable. Therefore all the bond angles are 1095 as per the VSEPR theory.

Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Chemistry Chemistry Labs

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Teaching Chemistry Organic Chemistry Study

Cyclohexane Chair Flip Summary Of How To Draw A Ring Flip Chemistry Mcat Study Student Learning
No comments for "Bond Angle of Cyclohexane"
Post a Comment